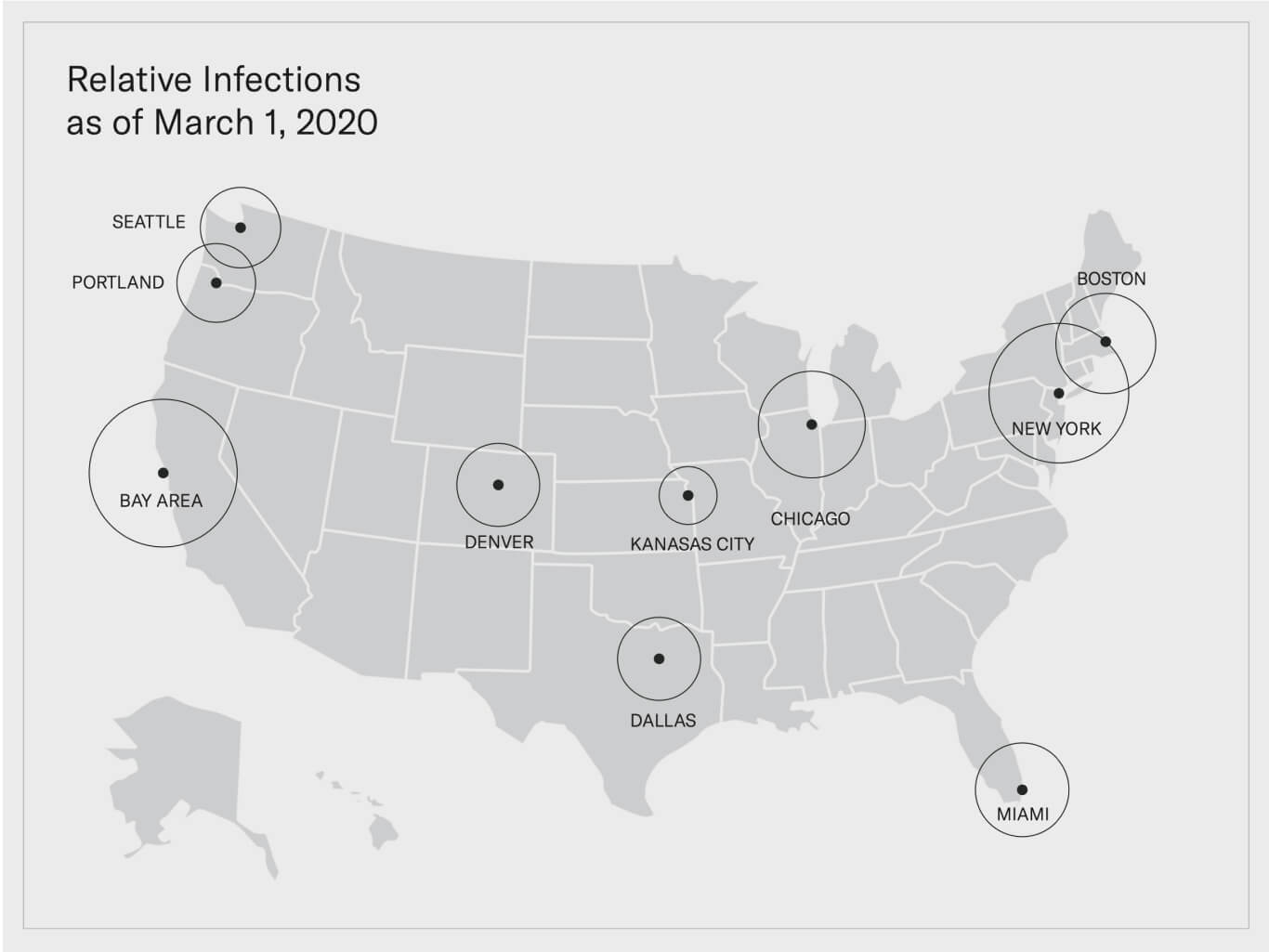
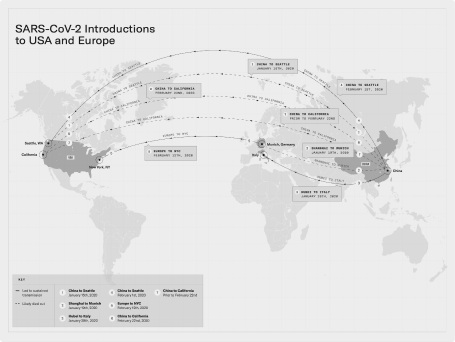
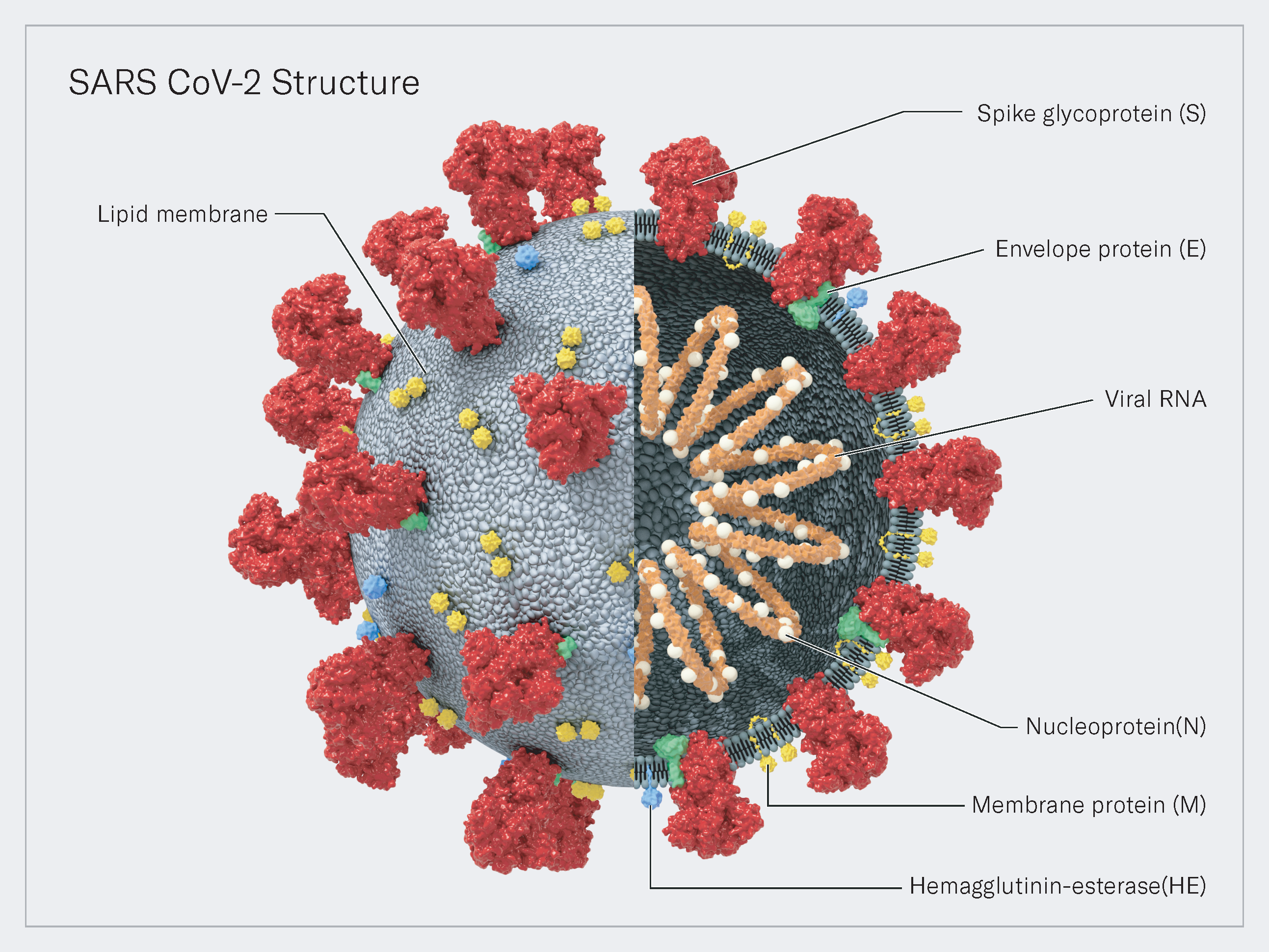
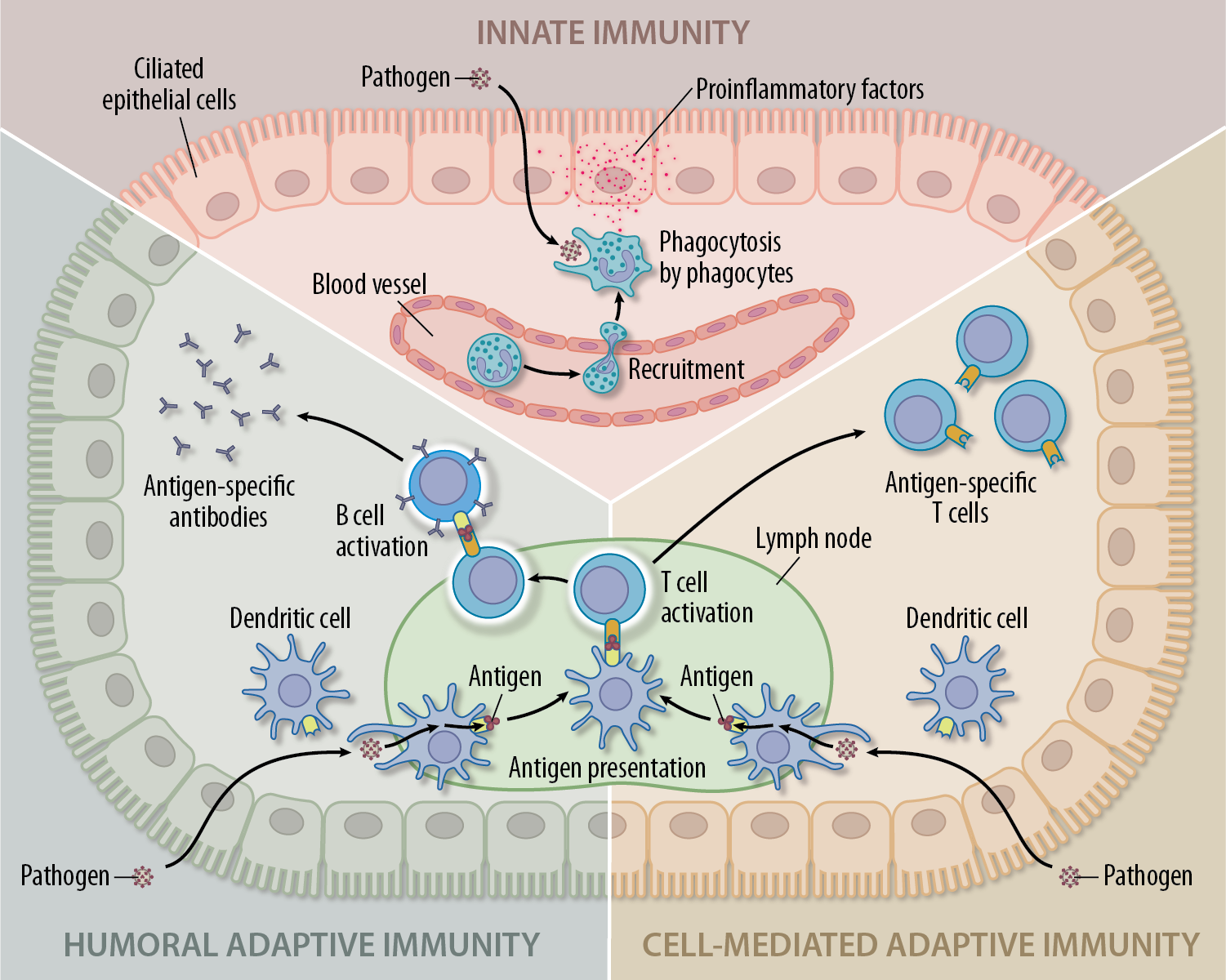
A roadmap developed and deployed during a time of crisis.
Accelerating Diagnostics in a Time of Crisis: The Response to COVID-19 and a Roadmap for Future Pandemics chronicles our nation’s effort to combat the SARS CoV-2 virus and bring millions of Covid-19 diagnostic tests to market in a period of less than a year.

About the Book
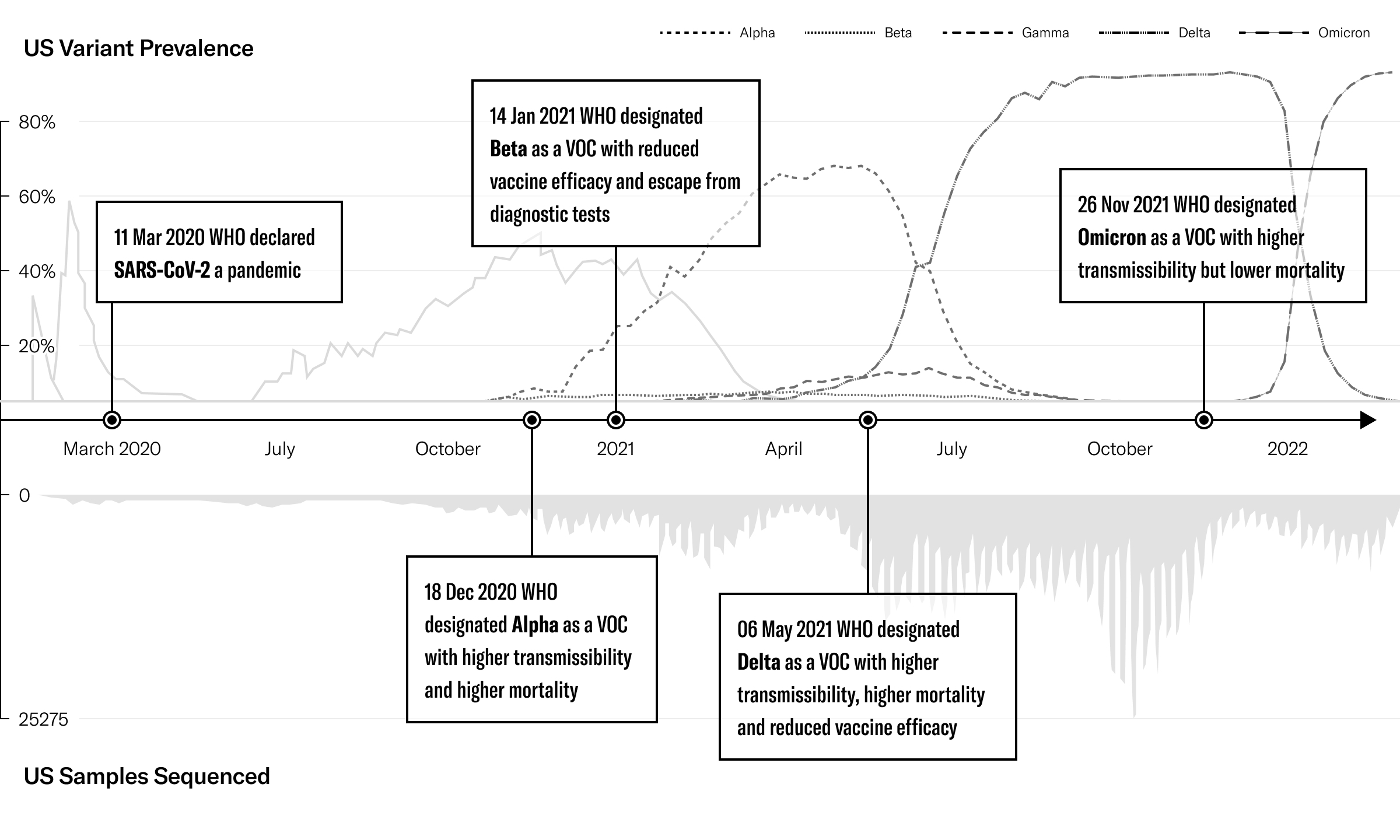
Despite the challenges inflicted by COVID-19 globally, the pandemic provided an opportunity to create and build an innovative and adaptive system that responded to each wave of the disease. This book provides a narrative of this response and draws from more than two years of data and protocols resulting in dozens of diagnostic tests and ground-breaking processes to address variant detection.
Co-authored by epidemiologists, virologists, immunologists, diagnosticians, molecular scientists, physicians, business executives, and other key opinion leaders who were real-time players in the pandemic response, this book is both a retrospective and prospective analysis, presenting lessons learned throughout the National Institute of Biomedical Imaging and Bioengineering’s (NIBIB) Rapid Acceleration of Diagnostics (RADx) Initiative.
The Pandemic Response Roadmap
Mapped to each chapter of the book, the roadmap provides a chronological encapsulation of tasks related to research, development, and commercialization of COVID-19 tests completed over a two-year period.
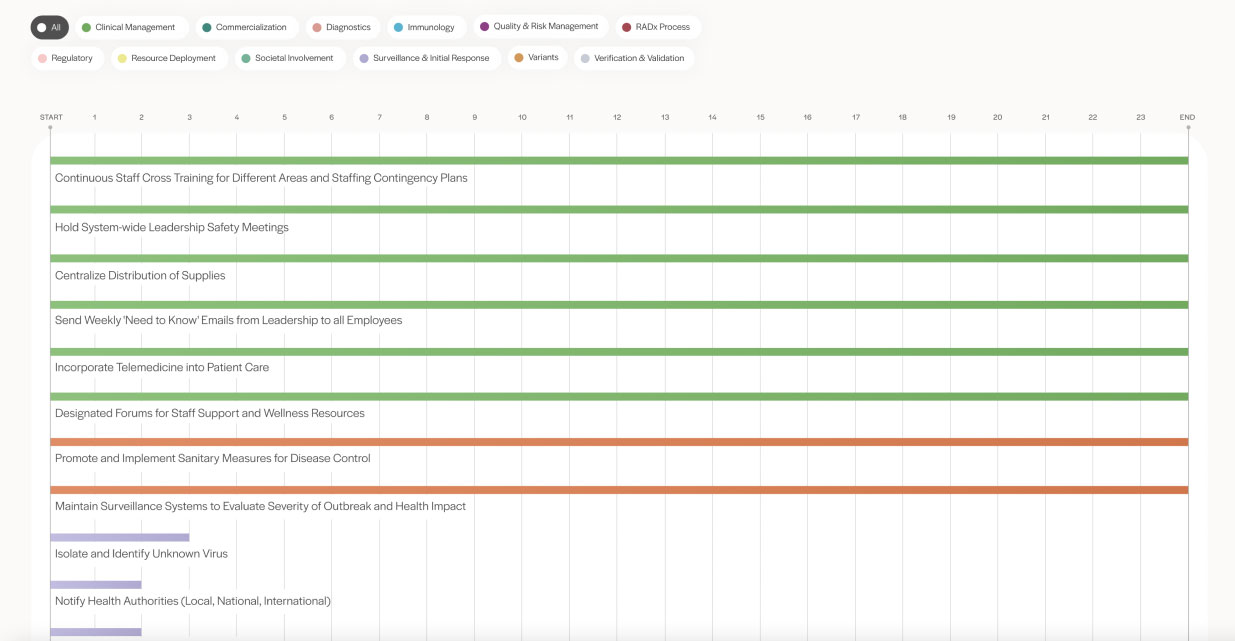
Conversational in tone but rich in science, data, and intricate technical detail, Accelerating Diagnostics in a Time of Crisis is written for lay and scientific audiences alike. The chapters collectively present a behind-the-scenes chronology of events and discoveries that occurred in the wake of the pandemic and provide a guide for a future pandemic response.

The Book
Accelerating Diagnostics in a Time of Crisis
Co-authored by epidemiologists, virologists, immunologists, diagnosticians, molecular scientists, and other key opinion leaders who were real-time players in the pandemic response, this book is both a retrospective and prospective analysis, presenting lessons learned throughout the NIBIB’s Rapid Acceleration of Diagnostics (RADx) Initiative.
Explore the graphics
View all of the book’s figures in greater details.
Related News
Insights into COVID-19: Perspectives on Drug Remedies and Host Cell Responses
Repurposing antiviral drugs, typically nucleoside analogs, has proven successful at inhibiting virus replication. This review summarizes current information regarding presents...
Critical care and pandemic preparedness and response
This review provides an introduction to an array of topics that pertain to different states of pandemic acuity: interpandemic preparedness,...
National governance and excess mortality due to COVID-19 in 213 countries: a retrospective analysis and perspectives on future pandemics
National governance may have influenced the response of institutions to the Covid-19 pandemic, being a key factor in preparing for...